January 14, 2026
iso 13485 certification costmedical device qmsiso 13485 audit feesqms certification cost
Understanding Your ISO 13485 Certification Cost
What's the real ISO 13485 certification cost? This guide breaks down every fee, hidden expense, and cost-saving strategy for medical device companies.
22 min readAI Gap Analysis

When you're looking at getting your medical device company ISO 13485 certified, the first question is always: "How much is this going to cost?" It’s not as simple as paying a single fee. For a smaller business, you're typically looking at a total investment between $20,000 and $50,000, covering everything from the audit itself to all the internal prep work.
Let's break down exactly where that money goes.
Decoding Your Total Investment in ISO 13485
Budgeting for ISO 13485 is a lot like planning a major renovation. You have the obvious costs—like the permit from the city (your final certificate)—but the real investment is in the foundation, the materials, and the skilled labor needed to get the job done right. In this case, that means building out your Quality Management System (QMS), training your team, and undergoing a series of rigorous audits.
The key to an accurate budget is understanding that your total cost is a mix of clear, external fees and the less obvious, but equally important, internal expenses.
Direct vs. Indirect Costs
Direct costs are the straightforward invoices you'll get from outside vendors. Think of the fees you pay the certification body and the auditors they send. These are relatively easy to anticipate and plug into a spreadsheet.
Indirect costs, on the other hand, are all about the internal resources you pour into the project. This is the time your team spends documenting procedures, fixing gaps, and managing the certification process. It also includes any new software you might need. These costs are often overlooked but can easily make up a huge chunk of your total spend.
This diagram gives you a clear visual of how your total investment splits between these two categories.
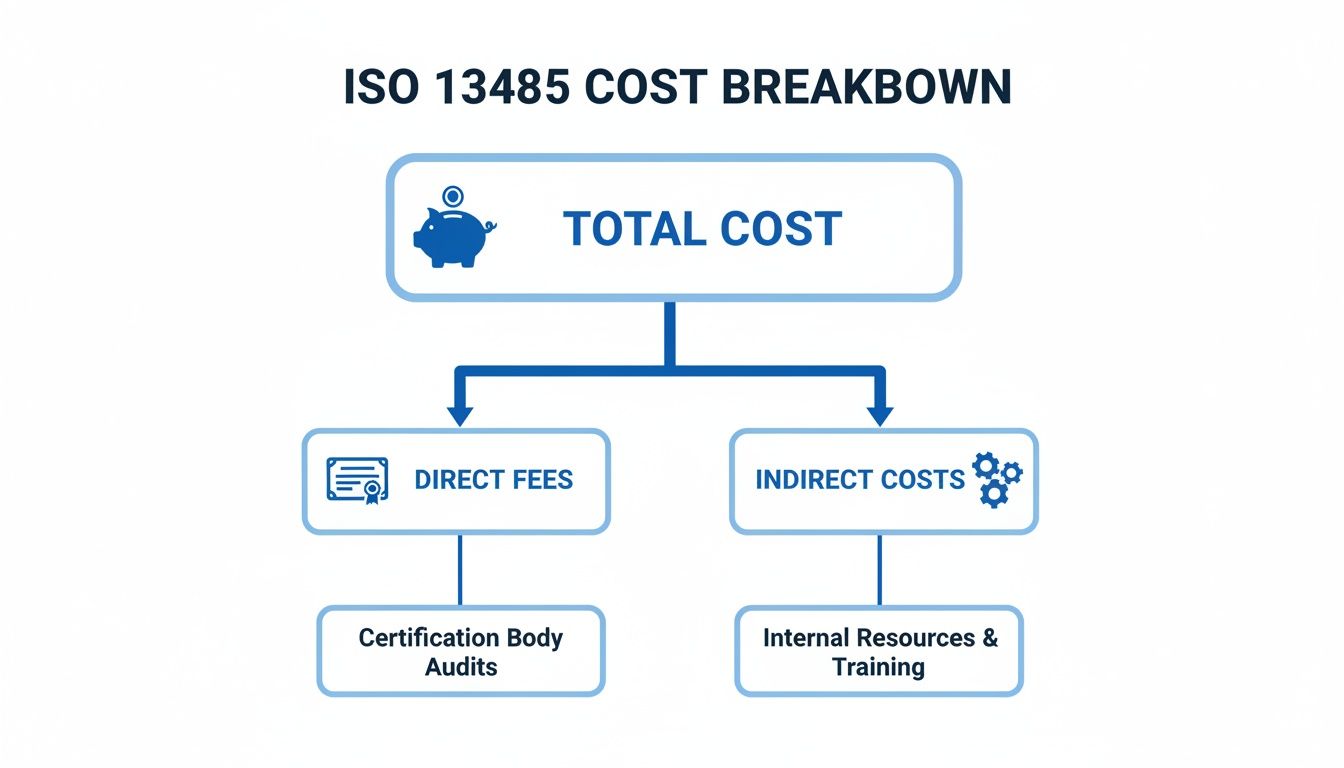
As you can see, what you spend internally to prepare is just as significant as the check you write for the certificate itself.
A High-Level Financial Snapshot
So, what do the actual numbers look like? Achieving ISO 13485 certification—the gold standard for medical device quality management—involves a few key external fees. Just the core certification process can cost a small business between $2,000 and $5,000, but that figure quickly grows when you add in all the preparation.
For example, the mandatory Stage 1 and Stage 2 audits, where auditors review your documentation and then visit on-site, typically run from $1,000 to $3,000, depending on your company's complexity. Issuing the final certificate tacks on another $500 to $1,000. You can find more detail on the factors that determine ISO 13485 certification cost from industry experts.
Key Takeaway: One of the biggest budgeting mistakes is seeing certification as a one-time fee. The smart approach is to budget for both the external "sticker price" and the deep internal investment needed to build a QMS that will actually pass the audit.
To help you get started, the table below provides a quick summary of the main cost components you'll need to account for.
At a Glance: ISO 13485 Cost Components
| Cost Category | Typical Price Range (Small Business) | Key Influencing Factors |
|---|---|---|
| Certification Body Fees | $5,000 - $15,000 | Company size, device complexity, number of locations |
| Consultant Fees | $10,000 - $30,000+ | Scope of work, QMS maturity, consultant experience |
| Internal Resources | $5,000 - $20,000+ | Staff hours, project management, gap remediation |
| Software & Tools | $1,000 - $5,000 (Annual) | Document management systems, QMS software |
Thinking in terms of these distinct categories helps you build a realistic budget and avoid any last-minute financial surprises on your path to certification.
Your External Audit and Certification Fees: What to Expect on the Invoice
When you start budgeting for ISO 13485, the fees from your chosen certification body are the most straightforward part of the puzzle. Think of it like this: you've built a house (your QMS), and now you need to pay for the final inspection and the official certificate of occupancy. These external costs are all about paying for the auditor's time and the administrative work to get that certificate on your wall.
The biggest driver here is the number of audit days required. The more complex your company, the more ground the auditor has to cover, and the bigger your invoice will be. Simple as that.
The Two-Stage Audit Process
Your certification isn't a single event. It's a two-part process, and you'll get billed for each stage. This approach is designed to catch major issues early before you invest in the full, intensive on-site review.
-
Stage 1 Audit (Documentation Review): This first step is a "desk audit," usually done remotely. The auditor pores over your QMS documentation—your quality manual, procedures, and policies—to make sure everything looks right on paper. The goal is to spot any glaring gaps before they move on. Expect this to cost between $2,000 and $4,000.
-
Stage 2 Audit (Implementation Audit): This is the main event. An auditor comes to your facility to see your QMS in action. They'll be walking the floor, talking to your team, and digging through records to confirm you're actually doing what your documentation says you do. Because it's more intensive, it's also more expensive, typically running from $4,000 to $8,000, sometimes more.
The more organized you are, the smoother—and potentially cheaper—this goes. Running through a detailed ISO 13485 audit readiness checklist beforehand is one of the smartest things you can do to prepare.
Key Factors That Influence Audit Costs
That final bill isn't just a number pulled from a hat. Certification bodies have a pretty standard formula for calculating the audit scope and duration, which all comes down to a few key variables.
Here’s what they’ll look at to build your quote:
- Company Size: The number of full-time employees is a huge factor. More people usually means more complex processes and more records for the auditor to check.
- Number of Locations: Every physical site you want included in the certification scope adds to the audit time. It also often means extra travel costs.
- Device Complexity: A company making simple Class I devices will have a much shorter audit than a company producing complex, life-sustaining implants.
Expert Insight: Implementation costs dominate the ISO 13485 certification landscape, hitting small companies with $8,000 to $16,000 for offsite work and climbing to $25,000+ for complex onsite efforts, especially in major markets like North America and the EU where regulatory scrutiny is intense. Consultation fees alone can range from $2,000-$4,000 offsite or $7,000-$11,000 onsite, scaling with employee count and QMS maturity—firms with existing systems can save up to 40%.
Additional Certification Body Fees
The audit itself makes up the bulk of the cost, but it's not the whole story. When you get a quote, make sure it breaks down all the potential charges so you don't get hit with surprises.
Keep an eye out for these other common line items:
- Application or Registration Fee: A one-time administrative fee to get you in their system, usually $500 to $1,000.
- Certificate Issuance Fee: The charge for actually printing and sending you the official ISO 13485 certificate.
- Auditor Travel and Expenses: For the on-site Stage 2 audit, you'll be on the hook for the auditor's flight, hotel, and meals. Depending on where they're coming from, this can easily add another $1,500 to $3,000 to your total.
Accounting for Internal Preparation and Hidden Costs
The invoice you get from your certification body? That’s just the tip of the iceberg. The real investment, and the biggest wildcard in your total ISO 13485 cost, is what happens inside your own company.
Simply paying the auditor without doing the heavy lifting beforehand is like signing up for a marathon and then showing up on race day without having run a single mile. We all know how that ends. These "hidden" costs are almost entirely about your team's time, and every hour your people spend getting ready is a real, tangible expense.
The True Cost of Staff Time
Your team's biggest time-sink, by far, will be building out your Quality Management System (QMS). This isn't just about writing a few new documents; it's a fundamental shift in how you operate. The whole thing kicks off with a gap analysis, which is where you map out every single spot your current way of doing things doesn't meet the standard.
From there, the real work begins. Your team will have to:
- Fix the Gaps: This means rewriting procedures, designing new forms, and putting new controls in place for everything from product design to how you manage suppliers.
- Create the Documentation: Someone has to actually write the quality manual, the standard operating procedures (SOPs), and all the work instructions that become the backbone of your QMS. This takes serious time.
- Set Up Document Control: You need a rock-solid system to manage, review, approve, and track all these controlled documents. It's a project in its own right.
Getting a handle on this initial effort is critical. You can get a much clearer picture of what’s involved by reading our guide on the complete gap assessment process. This first step really sets the stage for everything—and every cost—that follows.
Budgeting for Essential Training
Training is another huge cost center that catches a lot of companies by surprise. It's not enough for your team to know what the new procedures are; they need to understand why they matter for compliance and patient safety. You can bet your auditor will be looking for proof that your people are competent and well-trained.
This isn't just about a quick onboarding session. You'll need to budget for specific, specialized training for people in key roles, especially your internal auditors. For perspective, after the 2016 revision of the standard, 60% of medtech companies said training was their number one hidden cost. A single course for an internal auditor can run $299-$389 per person, and getting a full team of 10-20 people up to speed can easily top $5,000.
Investing in Necessary Tools and Technology
Let's be honest: you can't run a modern QMS on a patchwork of spreadsheets and shared network folders. As your documentation piles up, just managing version control and approvals becomes a massive compliance risk. This is where you have to start thinking about investing in the right tools.
Key Takeaway: Trying to manage compliance manually is a false economy. The hours your team wastes digging for documents and untangling version histories will quickly cost you more than dedicated software.
For most companies, this means getting a Document Management System (DMS) or, even better, a full electronic QMS (eQMS) platform. These systems put document control on autopilot, make training records easy to track, and take a lot of the pain out of audit prep.
Here’s a rough idea of what to budget for:
- eQMS Software: For a small or mid-sized company, expect to pay anywhere from $5,000 to $15,000 a year on a subscription, based on how many users you have and the features you need.
- Calibration and Maintenance Software: You'll likely need tools to keep track of equipment schedules and maintenance records to meet specific clauses in the standard.
- Validated Software: If you use any software in your product design, testing, or manufacturing, you also have to budget for validation—the process of proving it works exactly as intended. This can be surprisingly complex and time-consuming.
When to Invest in an ISO 13485 Consultant
So, you've reached a fork in the road: do you hire an ISO 13485 consultant or tackle this beast in-house? This is a huge decision. It's not just another line item in the budget; it's a strategic choice between spending cash on expertise versus spending your team's most valuable resource—time.
Think of it this way: hiring a good consultant is like bringing in a seasoned guide to navigate a dense, unfamiliar jungle. They already know the terrain, where the traps are hidden, and the fastest path to your destination, which in this case is a successful audit. Making that investment can slash your timeline and help you dodge expensive mistakes that might lead to a failed audit and a ton of rework.
Clear Signals You Need an Expert
Some situations practically scream for professional help. If your team is already running on fumes, has zero direct experience with ISO 13485, or you're up against a non-negotiable deadline to get your product to market, a consultant stops being a luxury and starts looking like a necessity. Their know-how can mean the difference between a smooth ride and a long, frustrating slog.
You should seriously consider bringing in a consultant if any of these hit a little too close to home:
- Lack of Internal Expertise: Your team is phenomenal at designing medical devices, but they've never had to build a Quality Management System (QMS) from scratch.
- Aggressive Timelines: You need that certificate, and you need it fast. Maybe it's for an investor milestone or to crack a new market. Either way, there’s no time for a learning curve.
- Limited Bandwidth: Your key people are already drowning in their day jobs. They simply can't carve out the hundreds of hours it takes to properly implement a QMS.
An experienced consultant doesn’t just build documents; they transfer knowledge. This ensures your team can confidently manage and maintain the QMS long after the initial certification is complete, turning a one-time cost into a long-term asset.
Understanding Consultant Fee Structures
Once you start looking, you’ll see that consultants generally charge in two different ways. Getting a handle on these models is crucial for managing your ISO 13485 certification cost and finding a partner that fits your budget.
-
Project-Based Fees: This is a flat, fixed price for the whole shebang, from the initial gap assessment all the way through to audit support. It gives you budget certainty, but it can be rigid if your needs suddenly change. For a small to mid-sized company, you can expect project fees to fall anywhere from $10,000 to over $30,000.
-
Hourly or Daily Rates: This model gives you a lot more flexibility. You can bring a consultant in for surgical strikes—like reviewing a critical set of documents or training your internal auditors. Rates vary a lot based on experience, but a typical range is $150 to $400 per hour.
The Alternative In-House Approach
Of course, going it alone is always an option. Managing the process internally saves you the direct cash outlay for a consultant, but it demands a massive time commitment from your team. This can work if you have a dedicated quality manager who’s been through the ISO 13485 rodeo before and a team that can realistically absorb the extra workload.
The trade-off is crystal clear: you’re swapping a direct financial cost for a huge indirect cost in staff hours and a potentially much longer runway to certification.
Budgeting for Long-Term Maintenance and Audits
Getting that ISO 13485 certificate isn't the finish line. Far from it. Think of it more like the starting gun for a long-term commitment. Your certificate isn’t a trophy to stick on a shelf; it's a living thing that needs constant care and attention to stay valid. While the initial certification cost is a big hill to climb, it's the ongoing expenses that truly determine whether your Quality Management System (QMS) stays effective and compliant for the long haul.
One of the most common budgeting mistakes I see is companies forgetting about these recurring costs. The entire certification cycle is designed to ensure you’re continuously improving, and every stage of that cycle has its own price tag.
The Annual Surveillance Audit Cycle
Once you’re certified, you officially enter a three-year cycle overseen by your certification body. To keep your certificate active, you'll have to go through surveillance audits in year one and year two. These aren't quite as grueling as the initial Stage 2 audit, but they're definitely not a simple formality.
An auditor will come in—either on-site or remotely—and take a close look at key parts of your QMS. They’re checking to see if you’re actually following your own procedures, keeping good records, and properly handling any issues that have popped up.
So, what does this cost? A good rule of thumb is to budget roughly 30% to 50% of what you paid for your initial certification audit. If that first big audit cost you $10,000, you should plan on spending between $3,000 and $5,000 each year for these check-ups.
Planning for the Three-Year Recertification Audit
When you reach the end of that three-year cycle, it’s time for a full-blown recertification audit. This one is a much bigger deal, closer in scope and intensity to your original Stage 2 audit. The auditor will perform a deep dive into your entire QMS to make sure it’s still fully compliant with the ISO 13485 standard.
As you might guess, the cost reflects this. A recertification audit will typically run you anywhere from 70% to 100% of your original Stage 2 audit fee. This is a significant expense that you absolutely need to plan for well in advance.
Key Takeaway: The ongoing ISO 13485 certification cost is about more than just paying the auditor. A huge chunk of your budget has to be dedicated to the internal work required to be audit-ready every single day.
Internal Costs of QMS Maintenance
Beyond the checks you write to your certification body, there are the continuous internal costs you have to factor in. A healthy QMS isn't a "set it and forget it" system; it demands real resources and constant attention.
Your internal budget needs to cover things like:
- Ongoing Training: Every time a new person joins the team or a procedure is updated, you have to conduct and document training.
- Internal Audits: The standard requires you to audit yourself regularly, and that takes up a lot of valuable staff time.
- Management Reviews: These mandatory reviews require serious prep time and pull your leadership team away from other duties.
- CAPA Management: Investigating problems and implementing Corrective and Preventive Actions (CAPAs) requires dedicated people and hours.
These internal efforts really add up. When you combine the external surveillance audit fees with all the internal work, you should brace for your annual maintenance cost to be around 33% of your initial investment.
You can get a deeper sense of how recurring certification costs impact businesses, particularly as the market evolves. If you want to put some hard numbers to it, our simple MDR cost calculator is a great tool for forecasting your potential expenses.
Proven Strategies to Reduce Your Certification Costs
Getting ISO 13485 certified is a significant investment, but the final price tag isn't set in stone. With a bit of smart planning, you can bring the total ISO 13485 certification cost down considerably without ever compromising on the quality of your QMS. It’s all about moving beyond just accepting quotes and starting to actively hunt for efficiencies.
The core idea is simple: replace slow, expensive manual work with smarter, technology-driven solutions. Think about it this way—you could hire a large crew with shovels to dig a foundation, which is costly and takes forever. Or, you could rent an excavator and get the same job done in a day for a fraction of the price. The same logic applies to getting your QMS ready for certification.
Embrace Technology to Slash Consultant Hours
One of the biggest and most unpredictable expenses in any certification project is the time spent on gap analysis and finding evidence. Traditionally, this means a consultant (or your own team) manually sifts through hundreds or even thousands of pages of documents, painstakingly checking them against the standard and logging every single gap. It’s an incredibly time-consuming and expensive grind.
This is where modern tools can make a huge difference. An AI-powered gap analysis platform, for example, can automate this entire discovery process. Instead of paying a consultant thousands of dollars for manual review, you let the software do the heavy lifting in a tiny fraction of the time.
For anyone managing compliance at a small or mid-sized company, this is a game-changer. It’s a sobering fact that a staggering 70% of companies underestimate the work involved in implementation, leading to budgets blowing out by 20-30%. Tools that automate document review and pinpoint gaps can cut down manual prep time from months to just days, which drastically lowers that risk. You can dig deeper into how these factors impact the final certification cost to see the full picture.
This screenshot from an AI Gap Analysis tool shows exactly how it finds specific evidence inside your documents.
The platform links its findings directly to the exact page and paragraph in your source files, which means no more hours spent hunting for proof.
Key Insight: The single most effective way to cut your ISO 13485 cost is to minimize the billable hours you pay to external experts. By using AI to automate the initial evidence discovery, you turn a massive, unpredictable consulting fee into a small, fixed software cost.
The table below breaks down just how different these two approaches can be. It's a clear look at how you can swap old, expensive habits for new, cost-effective methods.
Cost Reduction Tactics: Traditional vs. Modern Approach
| Task | Traditional Method (High Cost) | Modern Method (Cost-Effective) | Estimated Savings |
|---|---|---|---|
| Gap Analysis | Manual review by a consultant or internal team over several weeks. High hourly rates. | AI-powered platform scans all documents and identifies gaps in a few hours. | $5,000 - $15,000+ |
| Evidence Gathering | Manually searching through documents, emails, and folders to find proof for each clause. | AI automatically links findings to specific evidence locations (page, paragraph). | $3,000 - $8,000+ |
| Document Control | Using spreadsheets or generic file-sharing. Prone to versioning errors and difficult to manage. | Centralized document control software with automated workflows and version history. | $1,000 - $4,000 in time saved |
| Team Training | On-site consultant-led training sessions. Includes travel and high day rates. | Self-paced online training modules and role-based learning paths. | $2,000 - $6,000+ |
| Remediation Tracking | Managing tasks and fixes via email chains and manual project plans. Easy to lose track. | Integrated task management system that assigns owners and tracks progress automatically. | $1,500 - $5,000 in efficiency |
As you can see, the savings from adopting a modern approach aren't just marginal—they're substantial. By investing in the right tools, you fundamentally change the cost structure of your certification project for the better.
Bundle Certifications for Audit Efficiency
If your organization is also looking at ISO 9001, bundling it with your ISO 13485 certification is a classic cost-saving move. ISO 13485 is built on the same framework as ISO 9001, so there’s a ton of overlap in requirements for things like documentation, management reviews, and resource planning.
When you schedule a combined or integrated audit, you unlock some serious savings:
- Fewer Audit Days: The auditor can check off the overlapping clauses for both standards at the same time, which means fewer on-site days you have to pay for.
- Lower Admin Fees: You'll typically pay a single set of administrative and certificate fees instead of two separate bills.
- Simpler Prep Work: Your internal team can focus on preparing one integrated management system instead of running two separate projects, saving a huge amount of time and effort.
This strategy really is like getting two certifications for not much more than the price of one and a half.
Optimize Your Audit Logistics
Finally, don't forget about the small logistical details—they can add up fast. Just being smart about how you schedule and manage the audit can help trim the final invoice.
Keep these practical steps in mind:
- Push for Remote Audits: For surveillance audits or even the Stage 1 document review, always ask your certification body if parts of the audit can be done remotely. This wipes out the auditor’s travel, hotel, and meal expenses, which can easily save you $1,500 to $3,000 per audit.
- Invest in Pre-Audit Training: The cleaner your QMS is before the auditor shows up, the faster and smoother the whole process will be. A little targeted training on internal auditing and common issues helps your team find and fix problems before they become official non-conformities that might require a costly follow-up visit.
- Organize Your Evidence: Have every document, record, and piece of evidence perfectly organized and ready to go. Every minute an auditor spends waiting for you to find a file is a minute you're paying for. A well-prepared team can literally shorten the audit and leaves a fantastic impression.
Your Top Questions About Certification Costs, Answered
Let's be honest—trying to pin down the financial side of ISO 13485 certification can feel like nailing jelly to a wall. There are a lot of moving parts. This section tackles the most common questions we hear, giving you straight answers to help you build a realistic budget.
How Does the Certification Timeline Impact the Total Cost?
Time really is money when it comes to certification. A longer timeline for getting your ISO 13485 certification almost always inflates the total cost, and it happens in a few sneaky ways.
First, every extra week or month means more internal staff hours are sunk into the project, pulling people away from their day jobs. If you've brought in a consultant, you can bet their meter is still running, potentially adding thousands to your bill for every month of delay.
The longer it takes to get certified, the more you pay in "opportunity cost"—the revenue you're missing out on by not having your certified product on the market. But be careful: a rushed timeline can also be expensive if it leads to sloppy work and a failed audit.
Can I Get a Free Quote for My ISO 13485 Certification?
Absolutely. Any reputable, accredited certification body will be happy to give you a free, no-strings-attached quote for their services.
But here's the catch: that quote will only cover their direct fees—the Stage 1 and Stage 2 audits, issuing the certificate, and maybe travel costs for the auditor. It's just one piece of the financial puzzle. The quote won't account for the internal heavy lifting, like staff time, training, new software, or the cost of a consultant. Use these quotes to compare your options, but never mistake them for the total project budget.
What Are the Biggest Financial Mistakes Companies Make?
Underestimation. Without a doubt, it's the single biggest financial trap we see. Companies tend to fixate on the auditor's invoice and completely forget about the enormous internal effort required.
Here are the most common financial blunders:
- Ignoring Staff Time: Forgetting to calculate the hours your team will pour into writing procedures, attending training, and sitting in meetings.
- Skipping a Proper Gap Analysis: Just jumping in without a clear roadmap is a recipe for wasted effort and rework, which sends costs soaring.
- Trying to Manage It All Manually: Relying on a mess of spreadsheets instead of dedicated QMS software often leads to costly mistakes and painful inefficiencies.
- Forgetting the Long-Term Costs: Failing to budget for the annual surveillance audits and the full recertification audit that comes up every three years.
Is ISO 13485 Worth It for a Small Startup?
For any medical device startup, ISO 13485 isn't just a "nice-to-have"—it's often the price of admission. While that initial investment can feel daunting, it's what builds the foundation of quality that will pay you back for years.
Certification is a powerful signal to investors, partners, and regulators that you are serious about patient safety and compliance. It unlocks doors to global markets, especially in places like Europe where it's tightly woven into the regulatory approval process. For a startup, that cost is a direct investment in credibility and market access.
Ready to slash the time and cost of your gap analysis? AI Gap Analysis automates evidence discovery, replacing thousands in manual consultant fees with a simple, predictable platform. Find every gap in your documentation in hours, not weeks, and get audit-ready faster. Learn more and get started at https://ai-gap-analysis.com.